Chapter 1
Assessing 2019, and then the rupture of the COVID-19 pandemic
The medtech industry’s most recent financial performance, financing and M&A data show reasons for optimism in a disruptive, uncertain time.
Throughout the 2010s, the medtech industry maintained its solid financial performance year after year. While the financial crisis of 2007 and 2008 was a heavy blow to medtech and many other industries, the following decade witnessed a medtech resurgence due to strong fundamentals and investors’ high confidence in the sector. Though annual growth in revenues had yet to recapture the heights of the early years of the 21st century, 2019 was another solid year, particularly in R&D growth.
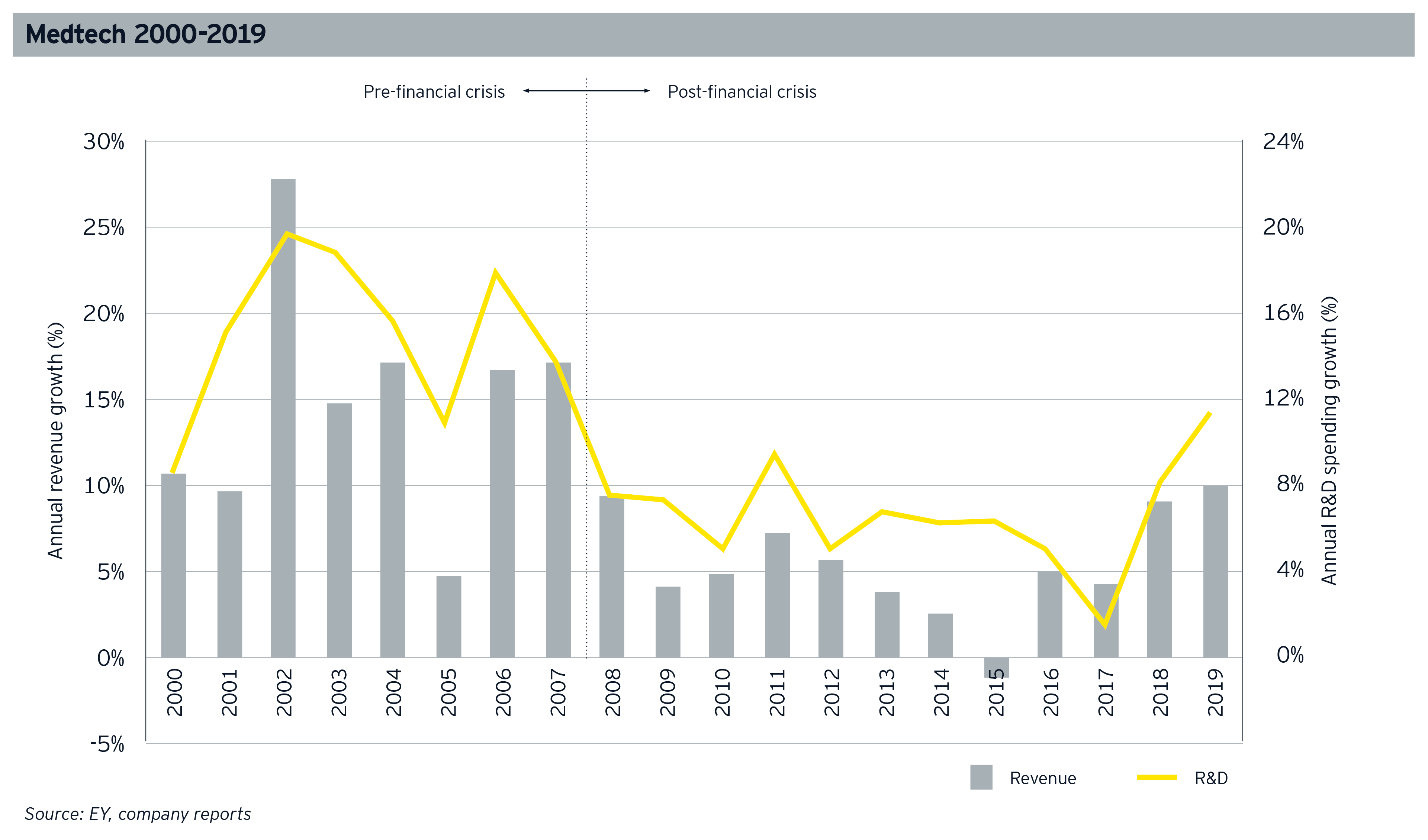
Though we don’t have full-year 2020 financial data to assess the impact of the pandemic (and the socioeconomic chaos it has brought in its wake) on medtech, we can already recognize that the industry’s financials in 2020 will look nothing like 2019 or any other year over the previous decade.
An analysis of Q1 and Q2 2020 financial reporting indicates that roughly two-thirds of US commercial leaders (pure-play medtech companies with more than US$500 million in annual revenue) and conglomerates have experienced an aggregate revenue decline of 5%. However, this figure conceals wide variations.
Among companies focused on elective procedures, the impact has been higher, as patients have stayed away from hospitals where the COVID-19 pandemic dominated clinical priorities in the second quarter of 2020. By contrast, companies focused on diagnostics saw toplines rise significantly with the heightened demand the pandemic brought.
Is medtech in a position to ride out the disruptions of 2020 and regain or even surpass its performance across the 2010s? Examining the industry’s most recent financial performance, financing and M&A data gives us a mostly affirmative answer — though not without some underlying causes for concern, too.
Medtech valuations rise again in 2020
Among medtech’s key metrics in 2020 to date, investor confidence stands out. Though medtech’s valuations fell along with the broader market (bottoming out in late March 2020), they recovered strongly in the subsequent months. By the end of August 2020, medtech’s valuations were up 50% compared to January 2019, much stronger than the rebound for broader composite indices such as the New York Stock Exchange and the S&P 500 (up 15% and 40%, respectively, over the same period).
Digital health companies rebounded even more strongly — up 65%, likely due to investor excitement over enhanced use of virtual health and other remote technologies during the COVID-19 pandemic But medtech’s commercial and noncommercial leaders both comfortably outperformed big pharmaceutical companies (which saw valuations rise 18% compared to January 2018) and biotech companies (up 40%).
A key driver of medtech’s high valuations was the non-imaging diagnostics segment, whose valuations rose 116% between January 2019 and August 2020, more than twice as much as for any other segment. In part, this reflects the urgent need for new diagnostic tools to combat the COVID-19 pandemic: the valuation of one diagnostics firm, Quidel, jumped 331% when the U.S. Food and Drug Administration (FDA) gave the company an emergency use authorization (EUA) for its rapid point-of-care test for detecting COVID-19 infection.
The COVID-19 pandemic could act as a growth driver for non-imaging diagnostics in the longer term as well. For example, Exact Sciences’ revenue surged 93% to US$876 million as use of its Cologuard at-home colon cancer tests doubled to 1.7 million.
Financing: major players load up on debt as startups face uncertainty
Medtech’s financing levels more than doubled to a record US$57.1 billion in the 12-month period from July 2019 to June 2020, compared with the previous 12 months. Over 40% of this dramatic growth was a result of US$35.6 billion of public debt financing, fueled by historically low interest rates. In fact, a record 18 companies raised US$500 million or more, with Thermo Fisher Scientific alone accounting for US$9.2 billion of the total.
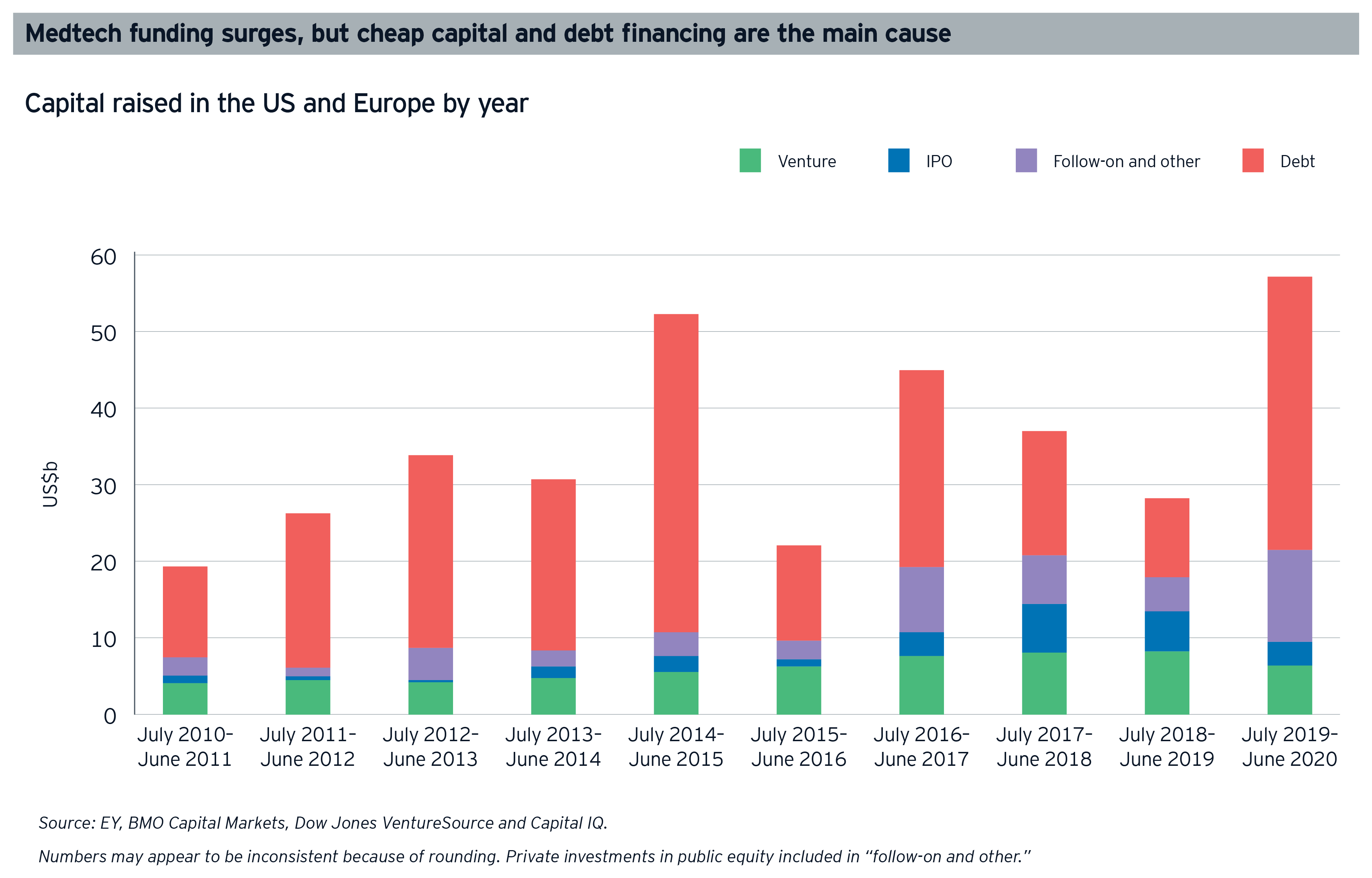
However, both debt and follow-on fundraising (about 23% of the period funding total) was driven by medtech’s bigger players, rather than the smaller companies that form a key component of the industry’s R&D engine. Overall “innovation capital” (money raised by the industry’s noncommercial leaders) slid to US$18.4 billion, accounting for only 32% of total funding (down from the previous 10-year average of 47%).
The IPO and venture capital (VC) fundraising channels saw less activity than debt and follow-on financing over the same 12-month period, which presents a challenging landscape for early-stage companies reliant on these financing options. And while the US$3.2 billion total for IPOs was the third highest on record, just 3 deals constituted roughly 85% of that volume, and the number of deals (14) was the lowest in a decade. Most of the IPO volume in the 12-month period considered here was concentrated in Q3 2019, before the COVID-19 pandemic had an impact. IPO activity fell precipitously in the first two quarters of 2020.
M&A: will the pendulum swing back in 2021?
The disruptive impact of the COVID-19 outbreak is particularly evident in the industry’s M&A performance, with M&A expenditures from July 2019 to June 2020 plunging 60% to US$27.1 billion compared to the previous 12-month period. An already-low total deal value was further reduced when Thermo Fisher Scientific was rebuffed on its proposed US$12.5 billion acquisition of Qiagen in August 2020. Focused on molecular diagnostics, including in infectious disease, Qiagen saw its operating income jump 84% in the first six months of 2020 due to the impact of the COVID-19 pandemic, leaving its shareholders reluctant to accept Thermo Fisher Scientific’s enhanced offer.
The next-biggest deal – Stryker’s US$5.4 billion proposed acquisition of orthopedic company Wright Medical – is under regulatory review in the US and the UK as of September 2020. However, the impact on M&A is not confined to the fall in such “megadeals” (those worth over US$10 billion): the total value of non-megadeals has also dropped 41%, while the average deal value across the industry shrank to US$167 million (from US$463 million in the previous year).
The slowdown in M&A, IPOs and VC funding raises concerns that a major source of innovation will disproportionately impact startups and small companies that are reliant on this capital. To sustain the cycle of innovation, larger medtech companies may need to consider other approaches, such as partnerships, incubators and more milestone payments (a strategy these companies frequently employed during the aftermath of the 2007 and 2008 financial crisis).
There are signs, however, that the big medtech players may instead be contemplating a surge of acquisitions in the near future. A buyer’s market may be developing as smaller, and perhaps even midsize, companies question whether they can survive the economic uncertainty triggered by the COVID-19 pandemic. Meanwhile, as noted, large medtech companies have recapitalized through debt and follow-on offerings, and now have substantial M&A firepower.
Chapter 2
Opportunities from the crisis: looking to the next and beyond
The COVID-19 pandemic challenges have highlighted room for improvement in business models, supply chains and regulatory relationships.
The underlying drivers for medtech’s transformation have been increasingly evident in recent years, and in many respects, the COVID-19 pandemic has accelerated them and created more urgency. The rise of connected devices is drawing medtech into the internet of things and opening up new opportunities for data-driven improvements in clinical outcomes. Meanwhile, health care systems face growing cost constraints, and patient-consumers are increasingly demanding a more customer-centered health care experience.
Now, the industry has the chance to address its limitations and better position itself to thrive in the next and the beyond, in three key areas.
1. Changing business models
At the beginning of 2020, 80% of physicians in the US were not using virtual health in their patient interactions — but six months after that, 95% had increased their use of virtual technology, with 58% of them increasing it by over 50%, according to a recent EY survey. The movement toward virtualized, remote-operated business models for medical care is accelerating faster than anyone had imagined at the start of 2020.
The need for remote care of chronic diseases has suddenly become more relevant than ever before, and medtech is already at the forefront of delivering this new model, particularly with the rise of diagnostics and the integration of diagnostics with remote care delivery. Beyond chronic diseases, populations worldwide face the challenges of maintaining mental and physical health while being cut off from normal routines. In recognition of this, the FDA lowered barriers to bringing behavioral therapy devices to market in April 2020, with the aim of heading off a potential mental health crisis.
Yet not all business model change is based on virtualization of care. As new health challenges emerge, new breakthrough innovations are needed to address them: in 2020, medtech players have aimed not only to rapidly design, redesign or retrofit devices that can help with the COVID-19 crisis management, but also to bring forward innovative offerings that can address the crisis. For one recent example, consider ExThera Medical’s Seraph 100 Microbind Affinity Blood Filter, a first-in-class tool for reducing pathogens in the bloodstream, even before identification of the pathogen.
Efficient producers, too, have never been as vital as in 2020, with health systems worldwide needing commodity equipment, from protective personal equipment, ventilators and diagnostics to many other hospital basics, at scale and at speed. Efficient producers with robust and agile systems for manufacturing and distribution have been at the forefront of medtech’s efforts to mitigate the disruptions caused by the pandemic. Not least among these disruptions has been its impact on global supply chains.
2. Supply chain transformation
Supply chain issues for medtech didn’t begin in 2020. Industry insiders have in the past noted the inefficiencies associated with supply intermediaries and inflexible legacy systems, in addition to the lack of transparency for regulators and companies alike. Concerns around supply chain visibility and efficiency were already at the forefront of industry discussions, with companies considering the wider use of analytics to address these issues, reduce costs and better meet their customers’ changing demands.
Moreover, nationalistic criticism of globalized operating models also preceded the pandemic, and these tensions look set to continue no matter how the COVID-19 pandemic plays out from this point. Medtech companies are obliged to assume a disrupted global political environment for the foreseeable future.
Participants in an Ernst and Young LLP/AdvaMed medtech CEO roundtable said that companies in the industry are scaled for efficiency, not redundancy. One said that using digital technology and data could be used to track how much scale redundancy suppliers have and whether they can pivot toward greater production. In the longer term, the industry will need to accommodate the ongoing reality of travel restrictions between countries and even between US states. The industry also may need to bring some manufacturing capacity back to the US or Europe, to safeguard the supply base.
And as business models change, new supply chain problems also will arise. Consider the rise of remote care: in an “anytime, anywhere” model, how can medtech companies shift their supply chains to accommodate this change? Moreover, as medical devices become ever smarter and more reliant on software and data, this will increase the need for medtech companies to take a broader product life cycle approach to their devices.
3. A revolution in regulation
Whatever the lasting commercial impact of the pandemic, we can see already that it has transformed regulatory norms. In the US, concern over product supply has not only led to increased policymaker involvement in the supply chain, but also significantly cut in the barriers to market entry, with the FDA authorizing over 250 emergency use authorizations (EUAs) since February 2020. These EUAs cover many products, including in vitro diagnostics and other tests, personal protective equipment and ventilators, and equipment that can be repurposed as ventilators.
Related article
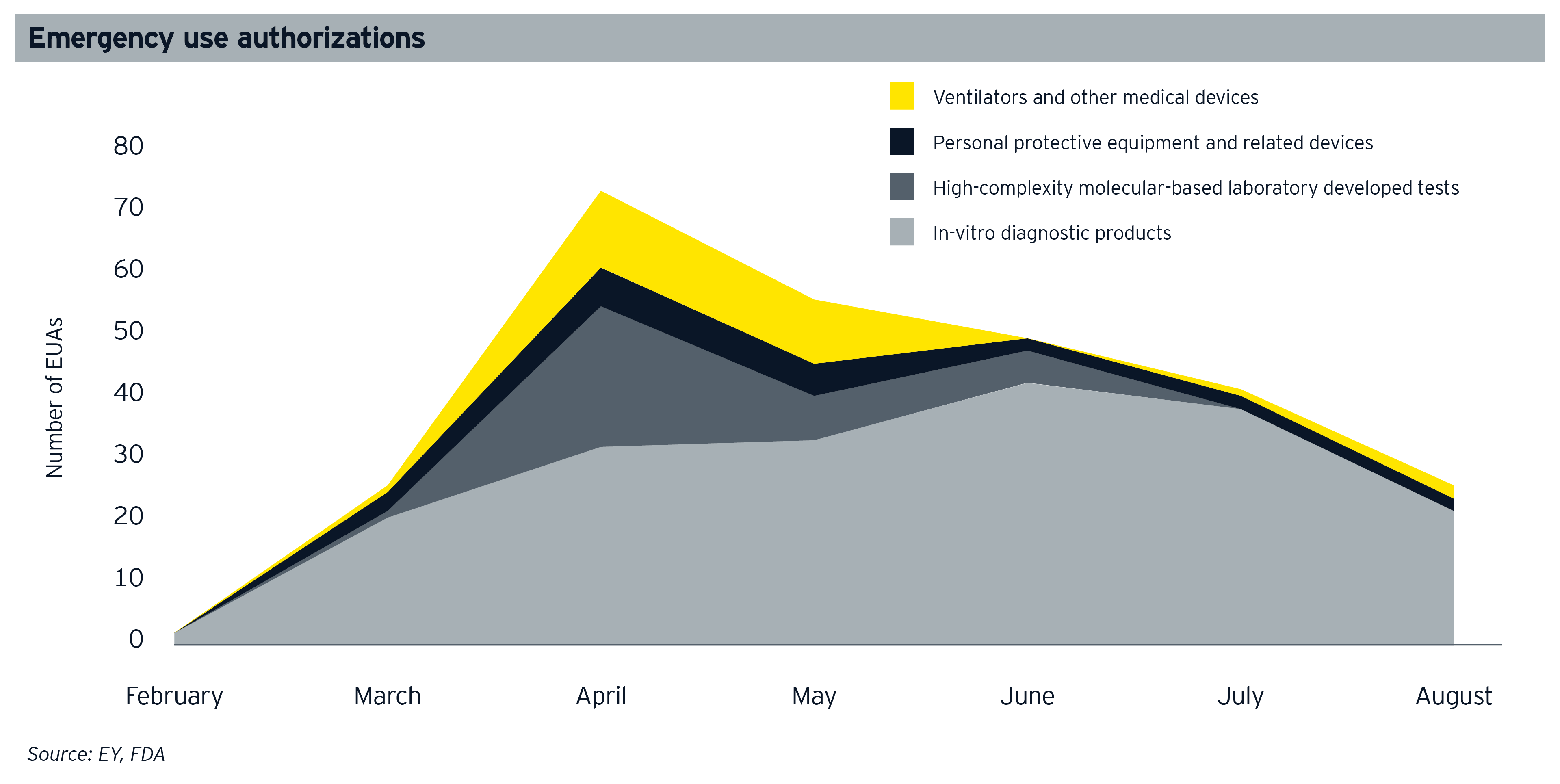
The loosening of regulatory conventions goes beyond EUAs and beyond the US, and it’s not only new products that have been accelerated into the medtech space but also new entrants — for example, multiple companies from outside the sector came together with medtech companies to meet the demand for vital equipment. Collaboration within the sector has also been facilitated by the regulators’ permissive attitude in 2020, with the U.S. Department of Justice and the Federal Trade Commission confirming that medical device suppliers can collaborate during the crisis without risking violation of antitrust laws, sharing capacity and expertise as needed.
The receptive attitude of the FDA and other regulatory bodies offers great scope for the industry to shape the dialogue about how it is regulated in future, and partnership is going to become an ever-more vital part of that conversation. Consider the FDA’s proposed approach to managing AI, which would see the agency moving away from regulating individual products toward a broader and more continuous assessment of companies as viable partners to bring software and analytics into the market. With the buy-in of regulators, medtech is well-placed to continue this transformation and usher in a new era of digital, data-driven smart devices that can potentially transform the industry.
Chapter 3
Digital health: moving from the horizon to the here and now
Challenges in this area have fallen one by one. Yet many remaining obstacles lie within the medtech industry itself. When will it change?
While the medtech industry has always been built on clinical data, the digital world evolving around us offers far broader, richer sources of data. From environmental data to lifestyle data and real-time data on biological processes captured inside and outside the body, we are living through a proliferation of data that has the potential to transform health care.
Stakeholders acknowledge this. They also recognize that the challenge now is not a lack of data, but a lack of data integration: the data being generated is trapped in silos, and there are multiple challenges to joining it together. In part, these challenges are technical, and the rapid progress of technology makes it likely that they will be overcome.
Other challenges are regulatory. We have seen how fast regulatory adaptation has happened during the COVID-19 crisis; now, we need to see that adaptation continue, to accommodate the use of data in ways that can transform health care.
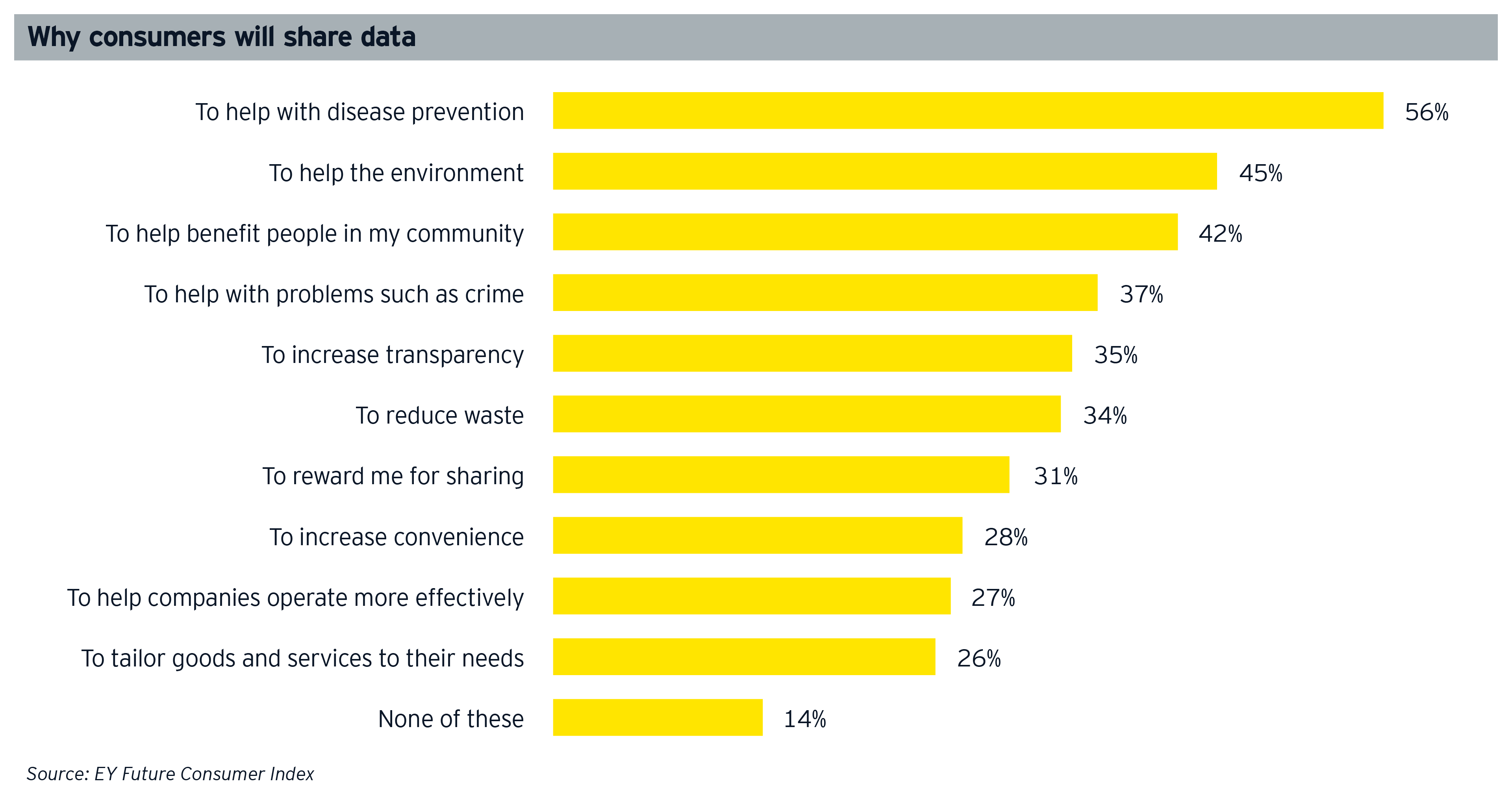
There is also the challenge of consumer willingness to share their data. Data privacy has presented an obstacle in the past, but the EY Future Consumer Index survey, performed in April 2020, suggests that 56% of consumers would make their personal data available if it helped to monitor and track an infection cluster, potentially opening an avenue for new business models.
Yet perhaps the biggest obstacle to data-driven transformation of the medtech business model has been the industry’s own reluctance to embrace change. While medical devices increasingly incorporate software and connectivity, many companies have hesitated to make significant investments into building the digital capabilities needed to access and use the ever-expanding wealth of real-world data.
It may be that the events of 2020 will conclusively demonstrate to medtech and the broader life sciences industry that digital acceleration is needed. Digital technologies are key to enabling the move toward remote care models for chronic disease and for health maintenance, and as such have been the focus of rapidly rising demand during the pandemic. While medtech valuations have performed more strongly than most other life sciences sectors, digital health has been even more favored by investors, with the Rock Health Digital Health Public Index rising 65% between January 2019 and August 2020.
Indeed, the largest M&A investment yet made in the digital health space occurred in August 2020 (outside the time period used in our M&A data in this report) when Teladoc Health announced that it will buy Livongo for US$18.5 billion — a potentially transformative move that creates a health tech giant and a new high benchmark for the valuation of digital health.
Livongo provides technologies to help people manage chronic conditions such as diabetes, and through this acquisition, they will be available across the 175 countries in which Teladoc Health is already active (instead of only in the US). It’s also notable that these two digital players have taken the initiative to create their new platform for themselves, rather than being united under a traditional medtech giant or Silicon Valley behemoth.
The final obstacle to embracing this digital transformation may lie within the culture of medtech companies themselves, which are used to viewing data as a proprietorial asset to be protected rather than a resource that can gain value from being shared. However, the COVID-19 crisis has allowed companies to work together without risking antitrust infringements. Greater collaboration — with competitors as well as customers — built on data can open future growth possibilities for medtech that will still be unfolding long after the pandemic is in the rearview mirror.
Summary
The medtech industry finds itself at a crossroads between crisis and opportunity, and between the past and whatever future we create as we emerge from the COVID-19 pandemic. New diagnostic tools and digital health enablers for at-home care and disease maintenance away from hospitals and clinics will drive growth today and long after the current crisis abates.

